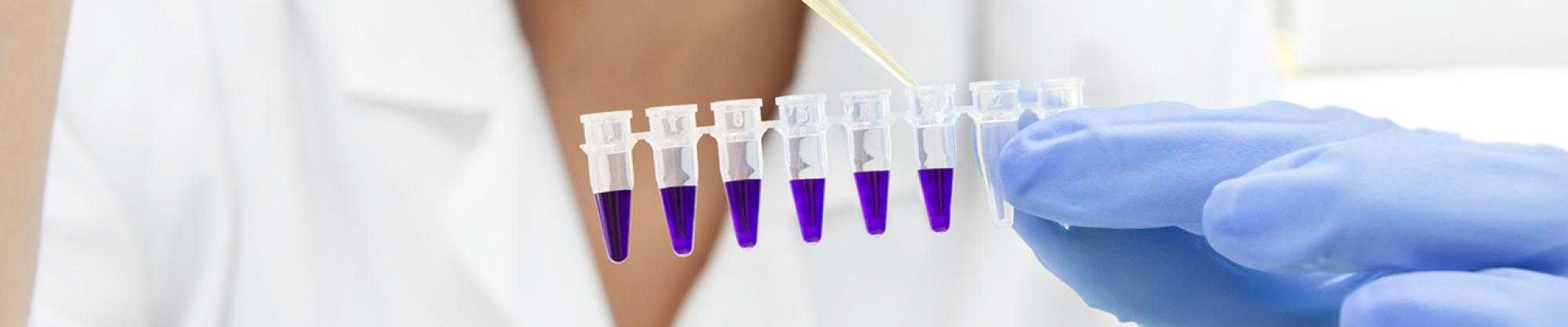
Protecting Production Integrity - Efficiently
From sterile disinfectants to clean-in-place wash down systems, ClearKlens solutions deliver dependable cleaning results while enhancing the efficiency and sustainability of your operation.
Developed to meet the specific needs of manufacturing facilities in the Life Sciences Industry, the comprehensive range of ClearKlens hygiene solutions can be relied on to protect your production integrity - efficiently.
Manufactured in a Controlled Environment to cGMP
The ClearKlens range is manufactured to cGMP principles (ISO 22716). Our sterile range is manufactured in cleanrooms accredited to the same standards as the cleanrooms the products are designed to be used in.
![]()
BPR Supported Formulation
The Biocidal Products Regulation (BPR) requires biocidal products to be registered with the European Union and will become the standard for all member states. As a significant investment per formulation, you can rest assured that Diversey ClearKlens is committed to the pharma sector and ensuring that we have a product range that is fit for the purpose.
![]()
Tested to Relevant Standards
ClearKlens biocides are tested against relevant standards to prove efficacy against bacteria, fungi and spores. Full test data is available on request.
![]()
Packaging Integrity
The ClearKlens sterile range is manufactured in sterile conditions to ensure the integrity of the product. Our packaging has been rigorously tested to ensure the integrity of the product is not compromised before or during use.
![]()
Standard Worldwide Formulations
The formulations of ClearKlens products are standard across the globe and are protected from change, ensuring no changes will be made unless for reasons beyond Diversey’s control and with extended notice.

Supported with Full Technical Documentation Package
The ClearKlens range is supplied with a document package containing relevant documentation including:
- Certificate of Analysis
- Certificate of Irradiation
- Certificate of Sterility
- Copies of the test data
- TOC and LD50 data
- HPLC test methods for residues
- Manufacturing specifications
- Process and packaging specifications where applicable
Please contact your Diversey representative to confirm local product range and availability products mentioned.